| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Case Report
Volume 5, Number 6, December 2015, pages 337-339
Cyclophosphamide-Induced Hyponatremia in a Patient With Diabetes Insipidus
Rachel A. Steinmana, Sara E. Schwaba, Kashif M. Munira, b
aDivision of Endocrinology, Diabetes and Nutrition, University of Maryland Medical Center, Baltimore, MD, USA
bCorresponding Author: Kashif M. Munir, Division of Endocrinology, Diabetes and Nutrition, University of Maryland School of Medicine, 827 Linden Avenue, Floor 2 South, Baltimore, MD 21201, USA
Manuscript accepted for publication November 19, 2015
Short title: Cyclophosphamide-Induced Hyponatremia
doi: http://dx.doi.org/10.14740/jem319w
| Abstract | ▴Top |
Cyclophosphamide has been previously observed to induce hyponatremia. The mechanism remains unclear. Cyclophosphamide may produce a syndrome of inappropriate anti-diuretic hormone-like phenomenon through impairment of the kidney’s ability to dilute urine. Whether cyclophosphamide or its metabolites have a direct effect on the kidney, a vasopressin-like effect on the kidney, or cause vasopressin release is unknown. A 29-year-old man with intracranial germinoma diagnosed at age 11 treated primarily with chemo-radiation developed a recurrence 17 years later and was noted to have panhypopituitarism with resultant central diabetes insipidus. He was admitted for chemotherapy with cisplatin, cyclophosphamide and mesna. His admission serum sodium was normal, but he became hyponatremic while undergoing chemotherapy with cyclophosphamide and mesna in conjunction with the initiation of intravenous hydration with 5% dextrose/0.45% normal saline despite withholding two doses of desmopressin (DDAVP). The sodium eventually normalized after administration of 20 mg intravenous furosemide. A similar episode occurred several weeks later, while again receiving cisplatin, cyclophosphamide and mesna chemotherapy. He again became hyponatremic despite receiving isotonic saline fluids and withholding DDAVP during cyclophosphamide treatment. Serum sodium did not improve with three doses of 10 mg intravenous furosemide but improved instead with sodium chloride tablets. The mechanism of cyclophosphamide-induced hyponatremia remains unknown. Given that this patient’s central diabetes insipidus prevents him from secreting increased amounts of anti-diuretic hormone, hyponatremia is likely induced by a direct nephrogenic effect of cyclophosphamide or its metabolites.
Keywords: Cyclophosphamide; Hyponatremia; Diabetes insipidus
| Introduction | ▴Top |
It is well known that cyclophosphamide can result in hyponatremia. The mechanism, however, by which cyclophosphamide induces hyponatremia is yet to be fully elucidated. There is one prior case report that discusses the anti-diuretic effects of cyclophosphamide in an 8-year-old girl with established central diabetes insipidus [1]. Our case of a 29-year-old gentleman also with central diabetes insipidus and resultant cyclophosphamide-induced hyponatremia adds to the limited literature and suggests that cyclophosphamide or its metabolites must have a direct renal effect resulting in hyponatremia.
| Case Report | ▴Top |
A 29-year-old man with a history of intracranial germinoma diagnosed at age 11 was primarily treated with chemo-radiation and subsequently developed a recurrence 17 years later. He developed panhypopituitarism with resulting central diabetes insipidus requiring desmopressin (DDAVP). He was admitted for chemotherapy with cisplatin, cyclophosphamide and mesna (Fig. 1). His admission serum sodium was 142 mmol/L (normal range: 136 - 145). His serum sodium declined slowly over the course of a few days to 135 mmol/L while receiving cisplatin and his home dose of DDAVP. A significant decline in serum sodium to 125 mmol/L occurred while undergoing chemotherapy with cyclophosphamide and mesna in conjunction with the initiation of intravenous hydration with 5% dextrose/0.45% normal saline despite withholding two doses of DDAVP. There was an accompanying decline in serum osmolality (osm) from 282 to 264 MoM/kg (normal range: 275 - 295). Urine sodium was 102 mmol/L and urine osm was 308 MoM/kg (50 - 1,200) with a corresponding serum sodium of 126 mmol/L, suggesting anti-aquaresis. With 20 mg intravenous furosemide, serum sodium improved to 137 mmol/L with an associated urine sodium of 43 mmol/L and urine osm of 132 MoM/kg. The serum sodium peaked at 142 mmol/L. The hyponatremia occurred within 6 h after administration of cyclophosphamide and lasted approximately 35 h.
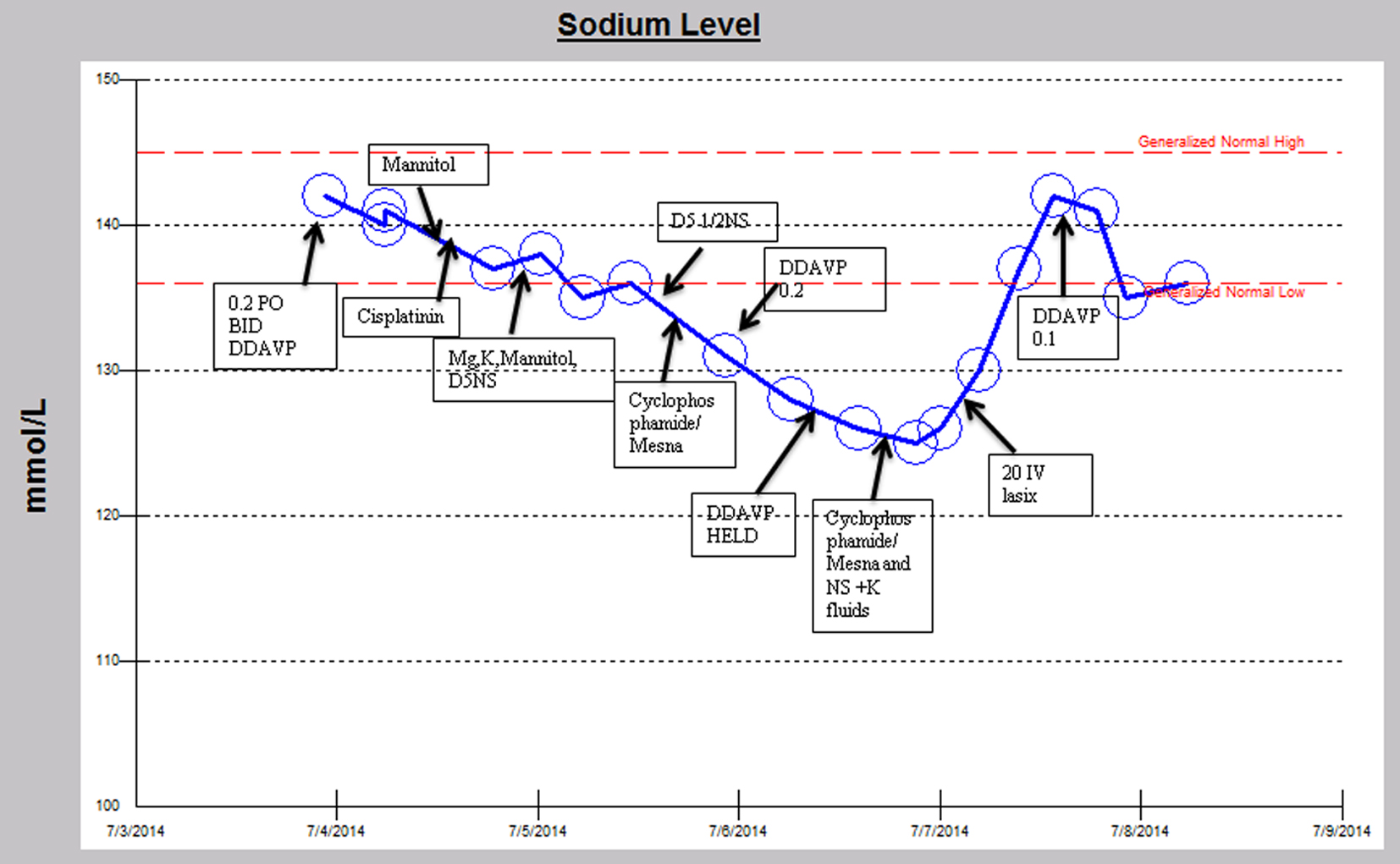 Click for large image | Figure 1. Sodium trend and relevant medication administration over the initial hospital course. |
A similar episode occurred several weeks later, while again receiving cisplatin, cyclophosphamide and mesna chemotherapy (Fig. 2). The patient’s serum sodium declined from 141 mmol/L to a nadir of 122 mmol/L and serum osmolality declined from 288 to 258 MoM/kg, despite receiving intravenous isotonic saline fluids and holding DDAVP after cyclophosphamide was initiated. The hyponatremia occurred within 6 h after receiving the cyclophosphamide. Urine osm was 654 MoM/kg concurrent with a serum sodium of 130 mmol/L suggesting anti-aquaresis. Subsequently in the hospital course, when the sodium reached a nadir of 122 mmol/L, the anti-diuretic hormone level was undetectable. Serum sodium did not improve with three doses of 10 mg intravenous furosemide but improved instead with sodium chloride tablets 4 g by mouth every 4 h to 134 mmol/L on discharge. The urine osm improved to 126 MoM/kg with a corresponding serum sodium increase to 132 mmol/L later in the hospital course during the upward trajectory of serum sodium. Hyponatremia resolved after approximately 55 h.
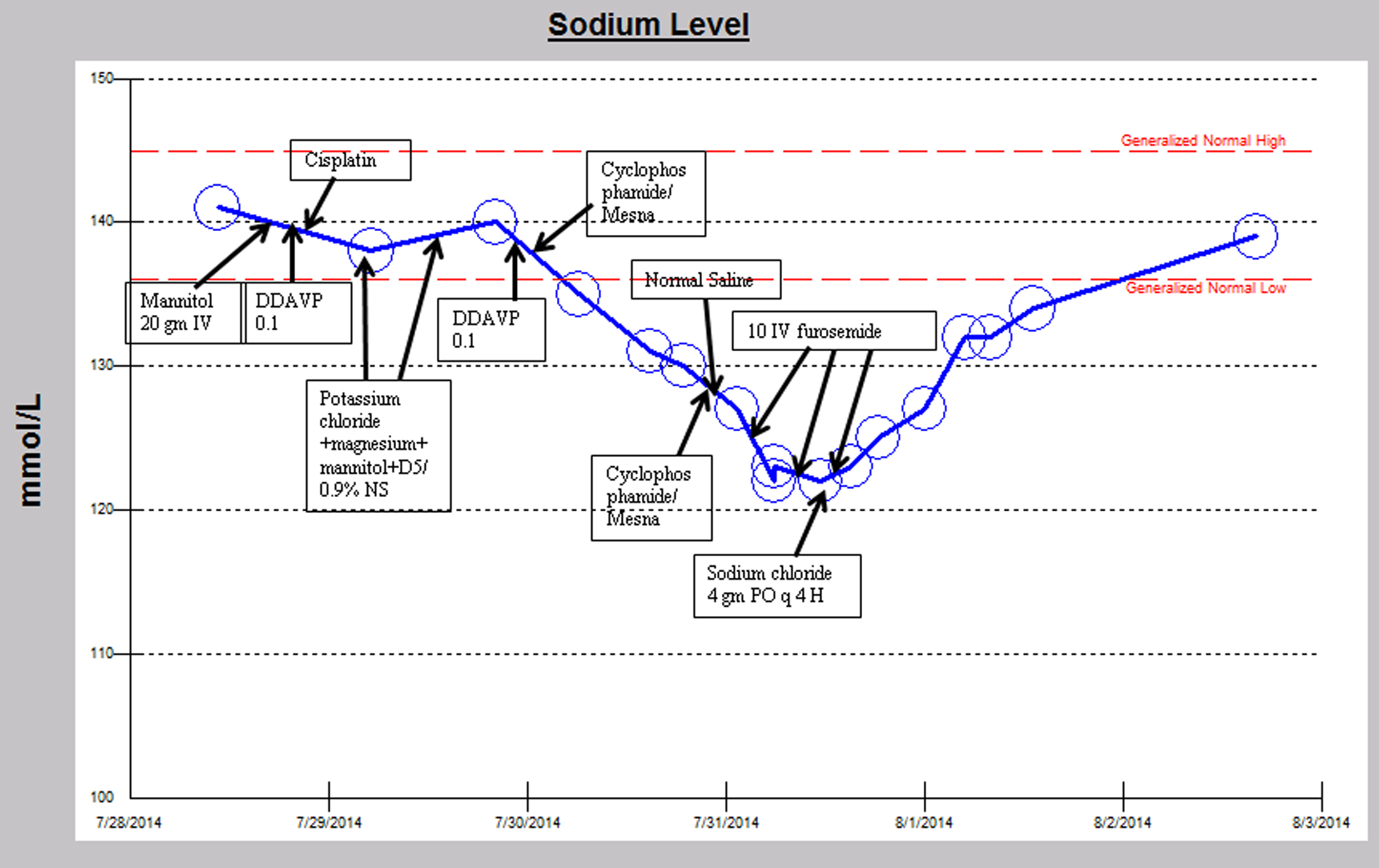 Click for large image | Figure 2. Sodium trend and relevant medication administration over the second hospital course. |
| Discussion | ▴Top |
Cyclophosphamide is an alkylating agent that has both antineoplastic and immunosuppressive properties. It is postulated to induce hyponatremia by limiting the renal excretion of water [2]. The exact mechanism by which cyclophosphamide produces this anti-diuretic effect is still under investigation. Some putative mechanisms are that cyclophosphamide or its metabolites induce vasopressin release, or directly act on the renal collecting tubules, or that cyclophosphamide metabolites have a vasopressin-like effect on the kidney [2-4]. A post-mortem exam in a patient with fatal, severe cyclophosphamide-induced hyponatremia showed loss of Herring’s bodies and degranulation of hypothalamic neurosecretory organelles, suggestive of syndrome of inappropriate anti-diuretic hormone (SIADH) [3, 5]. Some studies, including ours, however, have shown that plasma vasopressin levels are not elevated in patients who have received intravenous cyclophosphamide, thus suggesting more credibility to the theory that cyclophosphamide or its metabolites directly act on the kidney [2, 6]. In addition to our patient, it has previously been reported that an 8-year-old girl with central diabetes insipidus developed cyclophosphamide-induced antidiuresis [1], which suggests that cyclophosphamide does not cause vasopressin release since these patients would be unable to increase vasopressin levels. The undetectable anti-diuretic hormone level in our patient during an episode of hyponatremia also validates this point.
Recently, pre-clinical rodent studies have investigated the mechanism of this water retention and have suggested that cyclophosphamide may induce a drug-induced nephrogenic syndrome of inappropriate diuresis (NSIAD) by activating the vasopressin receptor 2 and upregulating aquaporin-2 channels and Na-K-2Cl cotransporter type 2, resulting in decreased renal water excretion [7]. Our patient, who is unable to secrete vasopressin and yet experienced significant cyclophosphamide-induced hyponatremia, supports this mechanism of an NSIAD that does not require vasopressin to induce the electrolyte derangement.
Studies have shown that cyclophosphamide-induced hyponatremia frequently occurs 4 to 12 h after administration of intravenous cyclophosphamide and resolves in approximately 24 h [2]. Our patient experienced hyponatremia within 6 h after cyclophosphamide initiation but it resolved after a much longer period of time (35 and 55 h). The prolonged duration of this hyponatremia is of unclear etiology. Perhaps, our patient was slower to metabolize this drug than other patients typically are or perhaps the furosemide, used with the aim of helping the hyponatremia, actually prolonged the hyponatremia. It is also possible that the DDAVP in our patient also prolonged the duration of hyponatremia. In the future, it may be beneficial to withhold DDAVP for a longer period of time prior to initiation of cyclophosphamide so as to take advantage of this patient’s diabetes insipidus and invariable progression to polyuria and hypernatremia.
While it is well known that cyclophosphamide can result in hyponatremia, the mechanism by which this occurs is yet to be fully elucidated. Our case report, to our knowledge, presents only the second case in the literature of a patient with diabetes insipidus who developed cyclophosphamide-induced hyponatremia, thus providing a human example that lends further credence to pre-clinical data. In the future, cyclophosphamide or its metabolites may even be considered as prototypes for the development of new drugs to treat diabetes insipidus.
| References | ▴Top |
- Campbell DM, Atkinson A, Gillis D, Sochett EB. Cyclophosphamide and water retention: mechanism revisited. J Pediatr Endocrinol Metab. 2000;13(6):673-675.
doi pubmed - Lee YC, Park JS, Lee CH, Bae SC, Kim IS, Kang CM, Kim GH. Hyponatraemia induced by low-dose intravenous pulse cyclophosphamide. Nephrol Dial Transplant. 2010;25(5):1520-1524.
doi pubmed - Bruining DM, van Roon EN, de Graaf H, Hoogendoorn M. Cyclophosphamide-induced symptomatic hyponatraemia. Neth J Med. 2011;69(4):192-195.
pubmed - Hwang SB, Lee HY, Kim HY, Lee ES, Bae JW. Life-threatening acute hyponatremia with generalized seizure induced by low-dose cyclophosphamide in a patient with breast cancer. J Breast Cancer. 2011;14(4):345-348.
doi pubmed - Harlow PJ, DeClerck YA, Shore NA, Ortega JA, Carranza A, Heuser E. A fatal case of inappropriate ADH secretion induced by cyclophosphamide therapy. Cancer. 1979;44(3):896-898.
doi - Bode U, Seif SM, Levine AS. Studies on the antidiuretic effect of cyclophosphamide: vasopressin release and sodium excretion. Med Pediatr Oncol. 1980;8(3):295-303.
doi pubmed - Kim S, Choi HJ, Jo CH, Park JS, Kwon TH, Kim GH. Cyclophosphamide-induced vasopressin-independent activation of aquaporin-2 in the rat kidney. Am J Physiol Renal Physiol. 2015;309(5):F474-483.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.









