| Journal of Endocrinology and Metabolism, ISSN 1923-2861 print, 1923-287X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Endocrinol Metab and Elmer Press Inc |
| Journal website http://www.jofem.org |
Original Article
Volume 4, Number 5-6, December 2014, pages 143-147
Tocilizumab Increases Serum Adiponectin and Reduces Serum Fatty Acid Binding Protein 4 in Patients With Rheumatoid Arthritis
Hayato Urushimaa, Yasuaki Sanadaa, Atsushi Ogatab, c, Masami Yoshidaa, Hsiaoyun Lina, Keisuke Hagiharad, Masashi Narazakie, Toshio Tanakab, c, Toshinori Itoa, Kazuhisa Maedaa, f
aDepartment of Complementary and Alternative Medicine, Osaka University Graduate School of Medicine, Osaka, Japan
bDepartment of Clinical Application of Biologics, Osaka University Graduate School of Medicine, Osaka, Japan
cDepartment of Immunopathology, WPI Immunology Frontier Research Center, Osaka University, Osaka, Japan
dDepartment of Kampo Medicine, Osaka University Graduate School of Medicine, Osaka, Japan
eDepartment of Respiratory Medicine, Allergy and Rheumatic Disease, Osaka University Graduate School of Medicine, Osaka, Japan
fCorresponding Author: Kazuhisa Maeda, Department of Complementary and Alternative Medicine, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita, Osaka, Japan
Manuscript accepted for publication December 02, 2014
Short title: IL-6 Ab Increases Adiponectin
doi: http://dx.doi.org/10.14740/jem251w
| Abstract | ▴Top |
Background: Recently tocilizumab, a humanized anti-interleukin-6 receptor antibody (IL-6R Ab), was clinically demonstrated to ameliorate metabolic syndrome. However, it is unknown whether blocking the IL-6R with tocilizumab directly impacts the adiponectin and fatty acid-binding protein 4 (FABP4) levels.
Methods: In this study, we measured the serum adiponectin and FABP4 levels in 18 patients with rheumatoid arthritis (RA) 3 months after treatment with tocilizumab.
Results: Our study revealed that treatment with tocilizumab decreased serum FABP4 levels and increased serum adiponectin levels in patients with RA. We also assessed the production of adipocytokines stimulated by IL-6R Ab using adipocyte precursors obtained from human fat tissue. Tocilizumab did not increase local adiponectin levels; however, the suppression of adiponectin secretion by IL-6 was completely abolished. Tocilizumab also directly suppressed FABP4 in human adipocytes.
Conclusion: This suggests that treatment with tocilizumab may be a novel approach to coordinately regulate adiponectin and FABP4 levels.
Keywords: Rheumatoid arthritis; IL-6; Adiponectin; FABP4; Tocilizumab
| Introduction | ▴Top |
Rheumatoid arthritis (RA) is a chronic inflammatory disease that affects approximately 1% of the general population and is associated with increased mortality, predominantly as a result of increased risk of cardiovascular disease (CVD) [1, 2]. Although the awareness of increased cardiovascular risk in patients with inflammatory diseases is increasing, the traditional risk factors for CVD in some patients remain suboptimally managed [3]. Recently, it was suggested that adipose tissue plays a role in chronic inflammatory diseases. It synthesizes and releases highly bioactive substances, including classical adipokines (such as leptin and adiponectin), and various pro-inflammatory cytokines (including tumor necrosis factor-α and interleukin-6 (IL-6)), which are collectively termed adipo(cyto)kines [4]. However, its ability to synthesize pro-inflammatory cytokines is not well understood.
Fatty acid-binding protein 4 (FABP4, also designated aP2 or adipocyte FABP) is expressed in adipocytes and other tissues and integrates inflammatory and metabolic responses [5, 6]. The expression of both FABP4 and adiponectin is regulated by peroxisome proliferator-activated receptor (PPAR)-γ [7]. However, the two proteins are differentially regulated because higher serum FABP4 levels [8, 9] and lower serum adiponectin levels [10, 11] have recently been found to be associated with metabolic syndrome (MetS) and CVD. Therefore, accumulating evidence suggests that the adipokine levels may act as biomarkers to dictate drug or dietary treatment strategies.
Tocilizumab, a humanized anti-IL-6 receptor antibody that blocks IL-6 signaling, is a novel therapeutic strategy for various autoimmune and inflammatory diseases, such as RA, Castleman’s disease, and juvenile idiopathic arthritis [12]. Although tocilizumab increased plasma adiponectin levels in patients with RA [13], the relationship between IL-6 and FABP4 is still controversial [14, 15], and the effects of tocilizumab on FABP4 are unknown.
The aim of the present study was to evaluate whether treatment with tocilizumab leads to changes in serum adipokine levels in patients with RA.
| Materials and Methods | ▴Top |
Patients
From July through December 2008, 16 patients (two males and 14 females) with active RA starting tocilizumab treatment were enrolled in this study between July and December 2008 at Osaka University Hospital. All patients had no history of medication use associated with PPAR-γ agonist (e.g., hypertension and diabetes) before enrolling in this study. They received a fixed dose of tocilizumab (8 mg/kg) in a single 1-h infusion every 4 weeks. Sixteen patients (89%) were also treated with oral prednisolone. Patient symptoms were assessed by disease activity score (DAS)-28, based on 28-joint counts for swelling and tenderness, tender joint count, and patient assessment. Serum FABP4 levels were measured using a human adipocyte FABP ELISA (BioVendor, Modrice, Czech Republic), and adiponectin levels were measured using an ELISA kit (Otsuka Pharmaceutical, Tokushima, Japan).
This study conformed to the Clinical Research Guideline of Osaka University Hospital and was approved by the institutional ethics committee. We obtained written informed consent to participate in this study from all patients.
Adipocyte culture and effects of tocilizumab on adipocytokine production in differentiated adipocytes
Preadipocytes were isolated from patients undergoing elective surgery who gave informed consent, as previously described [16]. Cells were differentiated into adipocytes by incubation in adipocyte differentiation medium (DM-2, Zen-bio®) at 37 °C with 5% CO2. After 8 days, cells were plated in fresh adipocyte medium (AM-1, Zen-bio®) and treated with drug-containing medium every 48 h. The effects of tocilizumab and 10 µmol/L pioglitazone hydrochloride on adiponectin and FABP4 secretion were analyzed on day 12 after 48 - 96 h of treatment.
Immunoblotting of FABP4
Human FABP4 expression was assessed by western blotting. In brief, lysates from human adipocytes were collected and separated on 16% SDS-PAGE gels, and transferred to PVDF membranes. Membranes were blocked for 1 h at room temperature in blocking buffer (5% skim milk in 10 mmol/L Tris, 100 mmol/L NaCl, 0.1% Tween 20, pH 7.5), and then incubated with anti-FABP4 antibody (A-FABP C-15, Santa Cruz) at a dilution of 1:1,000 for 1 h at room temperature. After washing (3 × 5 min in 1 × TBS-0.05% Tween), membranes were incubated with secondary anti-mouse (Dako) or anti-goat (Wako) antibodies conjugated to horseradish peroxidase. The immune complexes were detected using ECL Advanced Western Blot Detection System (GE Healthcare, Buckinghamshire, UK).
Statistical analysis
All results are presented as mean ± SEM. The differences in CRP, DAS-28, and serum adipokine levels before and after tocilizumab treatment were analyzed using paired t-test. Student’s t-test was used to compare the differences between the control and treatment groups. Values of P < 0.05 were considered to be statistically significant.
| Results | ▴Top |
The body weights of the patients were unchanged during the study. Treatment with tocilizumab for 3 months significantly suppressed the inflammatory process, demonstrated by a decrease in mean serum CRP levels from 1.8 ± 0.4 to 0.2 ± 0.1 mg/dL (P < 0.01). Levels of DAS-28 also improved significantly, from 3.9 ± 0.3 to 1.8 ± 0.4 (P < 0.01). The baseline serum adiponectin concentrations were 13.7 ± 1.7 μg/mL, which increased significantly to 18.0 ± 2.1 μg/mL (P < 0.01, Fig. 1A) after treatment with tocilizumab for 3 months. In contrast, baseline FABP4 concentrations were 31.3 ± 9.0 ng/mL, which decreased to 27.4 ± 9.4 ng/mL after treatment (P = 0.05, Fig. 1B). Although previous reports suggested that treatment with steroids significantly decreased serum adiponectin levels [17], oral prednisolone did not affect serum FABP4 or adiponectin levels in our study (data not shown).
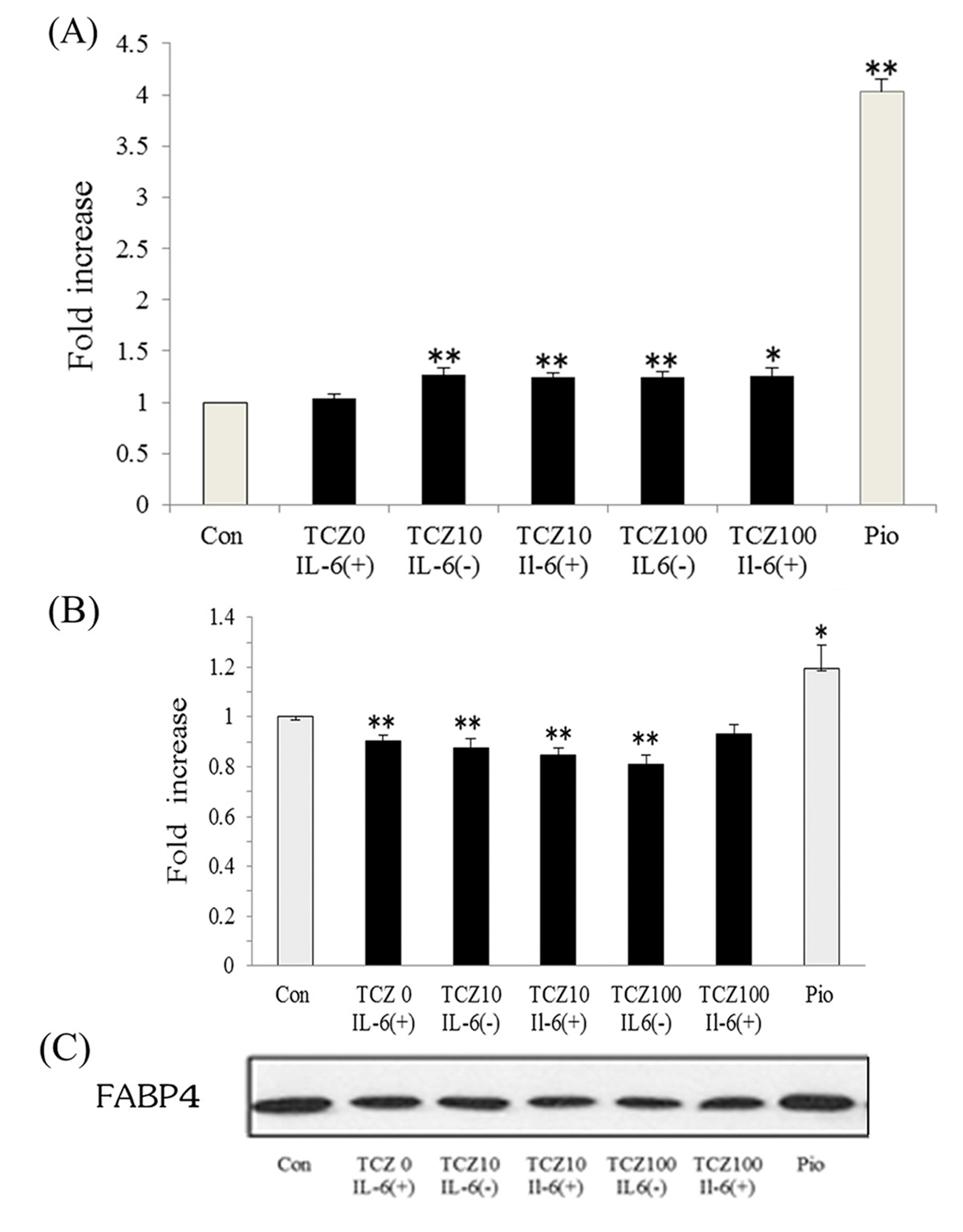
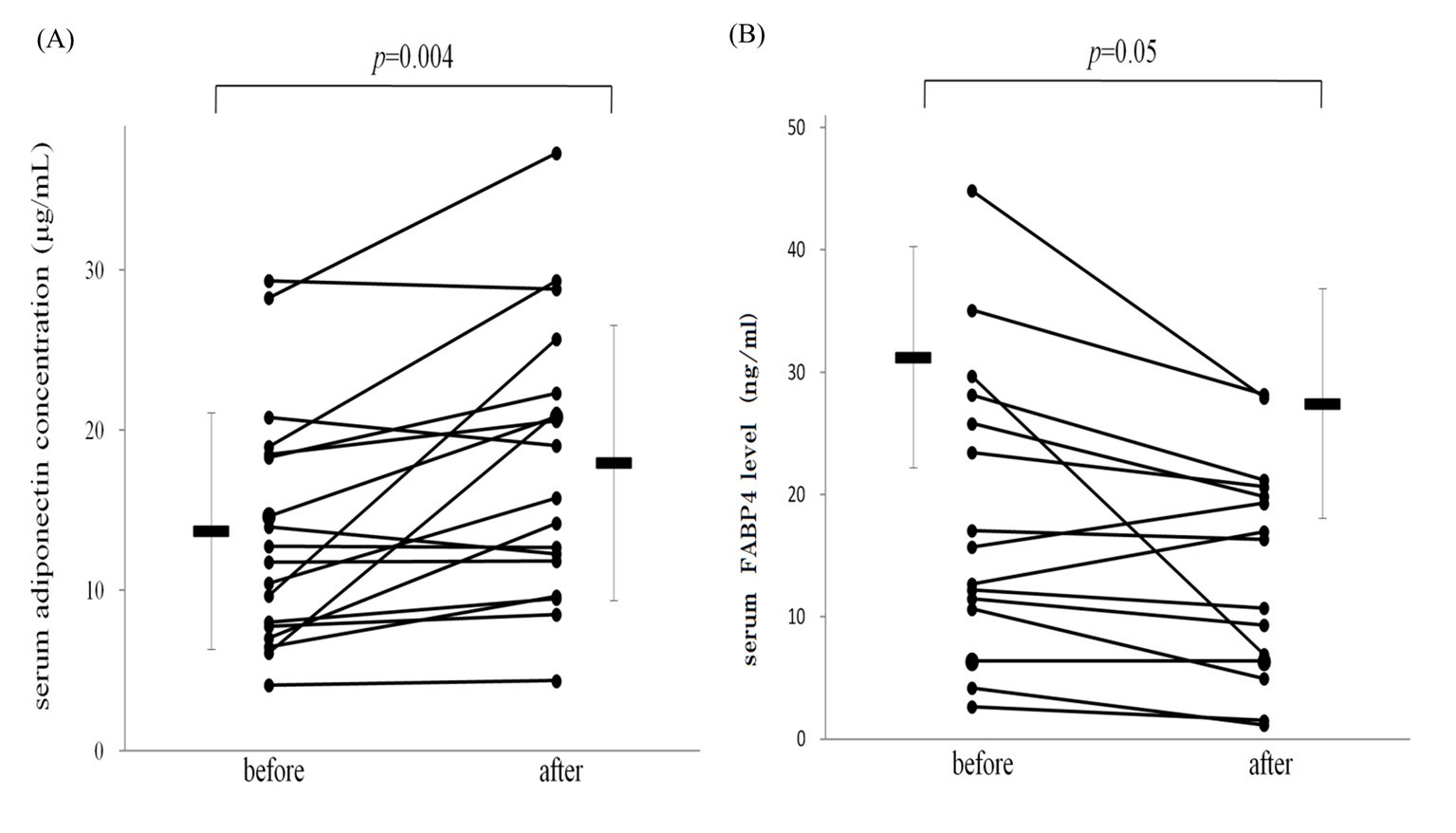 Figure 1. Treatment with tocilizumab increased serum adiponectin (A) and decreased fatty acid-binding protein 4 levels (B) in patients with RA. Bars represent mean ± SEM. Figure 1. Treatment with tocilizumab increased serum adiponectin (A) and decreased fatty acid-binding protein 4 levels (B) in patients with RA. Bars represent mean ± SEM. |
Next, the effect of IL-6 and tocilizumab on adiponectin and FABP4 was assessed in human adipocytes. After treatment of differentiated adipocytes with 100 µg/mL tocilizumab, adiponectin secretion was increased approximately 1.25 ± 0.04-fold (P < 0.01) compared with control. Tocilizumab treatment enhances adiponectin secretion by suppressing IL-6 (Fig. 2A). In contrast, FABP4 expression was decreased approximately 0.85 ± 0.03-fold (P < 0.01) in cells treated with tocilizumab compared with control. However, pioglitazone substantially increased FABP4 expression approximately 1.19 ± 0.10-fold (Fig. 2B, C).
| Discussion | ▴Top |
In this study, we found for the first time that treatment with tocilizumab, an IL-6R Ab, for 3 months reduced serum FABP4 levels in patients with RA. Both adiponectin and FABP4 are downstream targets of PPAR-γ in adipocytes. PPAR agonists such as telmisartan and thiazolidines induce the secretion of adiponectin [16] and simultaneously increase serum FABP4 levels [18, 19]. A previous study demonstrated olmesartan, which has little PPAR-γ activity, decreased serum FABP4 in patients with hypertension [20], while little is known about the effect of medication on FABP4 levels.
FABP4 promotes atherosclerotic diseases by acting on macrophages [21]. FABP4-deficient macrophages display defects in cholesterol accumulation and decreased pro-inflammatory cytokines TNFα, IL-6, and MCP-1 levels by reducing IκB kinase and NF-κB activity [22]. Although FABP4 is a circulating protein, the mechanism by which it enters the circulation is unknown. In both cross-sectional and prospective studies, serum FABP4 levels were positively correlated with lipid profiles, hyperglycemia, and non-alcoholic fatty liver diseases [23]. In addition, a 12-year community-based cohort study in a Chinese population indicated that plasma FABP4 levels were a strong predictor of CVD [24]. Because elevated FABP4 levels are a risk factor for CVD in patients with end-stage renal disease [8] and are correlated with numerous metabolic syndrome symptoms [9], the up-regulation of FABP4 may induce unfavorable side effects in patients treated with PPAR agonists.
Tocilizumab, a monoclonal antibody that blocks both membrane-bound and circulating IL-6R has anti-inflammatory actions that extend beyond reducing the concentrations of C-reactive protein and fibrinogen [25]. Patients with chronic inflammatory diseases, such as RA, are at increased risk of developing CVD [3], which is in part caused by increased IL-6 levels. Although further studies are required to confirm that these effects are also mediated by factors including macrophages and IL-6 in vivo, the present results suggest that changes in adiponectin and FABP4 levels reflect metabolic defects in adipose tissue and thus may be a useful biomarker of CVD in patients with RA.
Tocilizumab was identified as an agent that may help prevent coronary heart disease [26]. Because the suppression of adiponectin gene expression by IL-6 is mediated in part by p44/42 MAP kinase [27], the inhibition of this signaling pathway by tocilizumab may induce adiponectin secretion. The concurrent decrease in FABP4 levels and increase in adiponectin levels induced by tocilizumab may help prevent CVD and metabolic syndrome. However, additional studies are required to define the mechanism behind the differential effects of tocilizumab on FABP4 and adiponectin expression. For example, tocilizumab may act as a selective PPAR gamma modulator (SPPARM) [28] to reduce oxidative stress [29] or improve hypoxia [30]. However, SPPARM exhibited limited effects on FABP4 gene expression in mature 3T3-L1 adipocytes [28] and inhibited the differentiation of human preadipocytes compared with PPAR-γ agonists. SPPARM also displayed a diminished ability to induce FABP4 mRNA expression compared with rosiglitazone [29], whereas in human trophoblasts cultured under hypoxic conditions, the expression of FABP4 was enhanced [30].
In conclusion, tocilizumab treatment decreases FABP4 levels in patients with RA and could provide a novel therapeutic approach to prevent CVD.
Acknowledgement
We sincerely appreciate the cooperation of Miki Sakaue and Megumi Matsumoto from the Department of Complementary and Alternative Medicine, Osaka University Graduate School of Medicine, Osaka, Japan for accomplishing this research successfully.
| References | ▴Top |
- Dessein PH, Norton GR, Woodiwiss AJ, Joffe BI, Wolfe F. Influence of nonclassical cardiovascular risk factors on the accuracy of predicting subclinical atherosclerosis in rheumatoid arthritis. J Rheumatol. 2007;34(5):943-951.
pubmed - Solomon DH, Kremer J, Curtis JR, Hochberg MC, Reed G, Tsao P, Farkouh ME, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis. 2010;69(11):1920-1925.
doi pubmed - Kaplan MJ. Management of cardiovascular disease risk in chronic inflammatory disorders. Nat Rev Rheumatol. 2009;5(4):208-217.
doi pubmed - Neumann E, Frommer KW, Vasile M, Muller-Ladner U. Adipocytokines as driving forces in rheumatoid arthritis and related inflammatory diseases? Arthritis Rheum. 2011;63(5):1159-1169.
doi pubmed - Makowski L, Hotamisligil GS. Fatty acid binding proteins--the evolutionary crossroads of inflammatory and metabolic responses. J Nutr. 2004;134(9):2464S-2468S.
pubmed - Iso T, Maeda K, Hanaoka H, Suga T, Goto K, Syamsunarno MR, Hishiki T, et al. Capillary endothelial fatty acid binding proteins 4 and 5 play a critical role in fatty acid uptake in heart and skeletal muscle. Arterioscler Thromb Vasc Biol. 2013;33(11):2549-2557.
doi pubmed - Maeda K. Role of adiponectin and adipocyte fatty acid binding protein in the metabolic syndrome. Diabetes Res Clin Pract. 2007;77(Suppl 1):S17-22.
doi pubmed - Furuhashi M, Ishimura S, Ota H, Hayashi M, Nishitani T, Tanaka M, Yoshida H, et al. Serum fatty acid-binding protein 4 is a predictor of cardiovascular events in end-stage renal disease. PLoS One. 2011;6(11):e27356.
doi pubmed - Hsu BG, Chen YC, Lee RP, Lee CC, Lee CJ, Wang JH. Fasting serum level of fatty-acid-binding protein 4 positively correlates with metabolic syndrome in patients with coronary artery disease. Circ J. 2010;74(2):327-331.
doi pubmed - Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24(1):29-33.
doi pubmed - Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291(14):1730-1737.
doi pubmed - Schett G. Rheumatoid arthritis and multiple sclerosis: direful siblings, different strategies. FEBS Lett. 2011;585(23):3601-3602.
doi pubmed - Schultz O, Oberhauser F, Saech J, Rubbert-Roth A, Hahn M, Krone W, Laudes M. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS One. 2010;5(12):e14328.
doi pubmed - Lagathu C, Bastard JP, Auclair M, Maachi M, Capeau J, Caron M. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochem Biophys Res Commun. 2003;311(2):372-379.
doi pubmed - Gustafson B, Jack MM, Cushman SW, Smith U. Adiponectin gene activation by thiazolidinediones requires PPAR gamma 2, but not C/EBP alpha-evidence for differential regulation of the aP2 and adiponectin genes. Biochem Biophys Res Commun. 2003;308(4):933-939.
doi - Inomata-Kurashiki Y, Maeda K, Yoshioka E, Fukuhara A, Imagawa A, Otsuki M, Shimomura I. Measurement of adiponectin production from differentiated metabolic stem cells. Horm Metab Res. 2010;42(5):318-323.
doi pubmed - Harle P, Sarzi-Puttini P, Cutolo M, Straub RH. No change of serum levels of leptin and adiponectin during anti-tumour necrosis factor antibody treatment with adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65(7):970-971.
doi pubmed - Fujimoto M, Masuzaki H, Tanaka T, Yasue S, Tomita T, Okazawa K, Fujikura J, et al. An angiotensin II AT1 receptor antagonist, telmisartan augments glucose uptake and GLUT4 protein expression in 3T3-L1 adipocytes. FEBS Lett. 2004;576(3):492-497.
doi pubmed - Kim H, Haluzik M, Gavrilova O, Yakar S, Portas J, Sun H, Pajvani UB, et al. Thiazolidinediones improve insulin sensitivity in adipose tissue and reduce the hyperlipidaemia without affecting the hyperglycaemia in a transgenic model of type 2 diabetes. Diabetologia. 2004;47(12):2215-2225.
doi pubmed - Miyoshi T, Doi M, Hirohata S, Kamikawa S, Usui S, Ogawa H, Sakane K, et al. Olmesartan reduces arterial stiffness and serum adipocyte fatty acid-binding protein in hypertensive patients. Heart Vessels. 2011;26(4):408-413.
doi pubmed - Boord JB, Maeda K, Makowski L, Babaev VR, Fazio S, Linton MF, Hotamisligil GS. Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation. 2004;110(11):1492-1498.
doi pubmed - Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J Biol Chem. 2005;280(13):12888-12895.
doi pubmed - Koh JH, Shin YG, Nam SM, Lee MY, Chung CH, Shin JY. Serum adipocyte fatty acid-binding protein levels are associated with nonalcoholic fatty liver disease in type 2 diabetic patients. Diabetes Care. 2009;32(1):147-152.
doi pubmed - Chow WS, Tso AW, Xu A, Yuen MM, Fong CH, Lam TH, Lo SV, et al. Elevated circulating adipocyte-fatty acid binding protein levels predict incident cardiovascular events in a community-based cohort: a 12-year prospective study. J Am Heart Assoc. 2013;2(1):e004176.
doi pubmed - Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, Woodworth T, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58(10):2968-2980.
doi pubmed - Hingorani AD, Casas JP. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379(9822):1214-1224.
doi - Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, Paschke R. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;301(4):1045-1050.
doi - Balint BL, Nagy L. Selective modulators of PPAR activity as new therapeutic tools in metabolic diseases. Endocr Metab Immune Disord Drug Targets. 2006;6(1):33-43.
doi - Einstein M, Akiyama TE, Castriota GA, Wang CF, McKeever B, Mosley RT, Becker JW, et al. The differential interactions of peroxisome proliferator-activated receptor gamma ligands with Tyr473 is a physical basis for their unique biological activities. Mol Pharmacol. 2008;73(1):62-74.
doi pubmed - Biron-Shental T, Schaiff WT, Ratajczak CK, Bildirici I, Nelson DM, Sadovsky Y. Hypoxia regulates the expression of fatty acid-binding proteins in primary term human trophoblasts. Am J Obstet Gynecol. 2007;197(5):516 e511-516.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Endocrinology and Metabolism is published by Elmer Press Inc.